The Atom
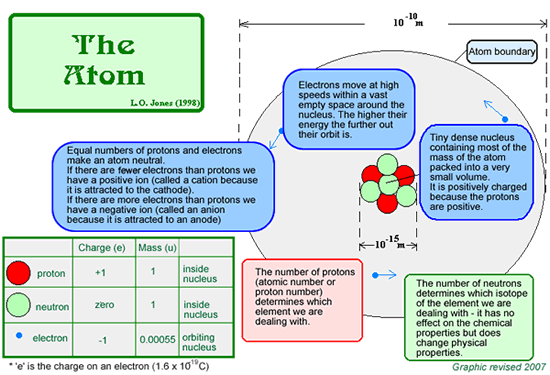
An atom is the smallest chemically indivisible part of an element - that means you can't split it by chemical (or physical) means into anything smaller - it takes a nuclear reaction to do it! All of the different types of atom known to exist in the universe are listed in the periodic table. Here is a simple diagram of the atom - you should KNOW this... including the sizes!
The Atomic Number is also called the Proton number of an atom (depending on which syllabus you follow). It is the number of protons in the nucleus - in chemistry it is usually called the atomic number - it tells us which element we are dealing with and determines the chemical properties of the element. The Atomic Mass is also called the Nucleon number of an atom (depending on which syllabus you follow). It is the number of nucleons in the nucleus - it is the sum of the number of protons and the number of neutrons - in chemistry it is generally called the atomic weight or atomic mass - it tells us which isotope of the element we are dealing with and has an effect of the physical properties - but not the chemical properties. A neutral atom has an equal number of protons and electrons.
The above diagram is not to scale. If it was drawn to scale and you had a nucleus of 1 cm diameter in the centre of your screen you would need a screen of 1km diameter to show the full graphic! Most of the atom is empty space!. Click here to find out how we know that the atom is like this.
For Chemists among you here is an interactive illustration of the orbital shapes of electrons. Click here to find out more about the nucleus and click here for an overview of the atom from PPARC.
You may want to find out more about the atom. These links will take you to more advanced pages on my site: |
Follow me...
|