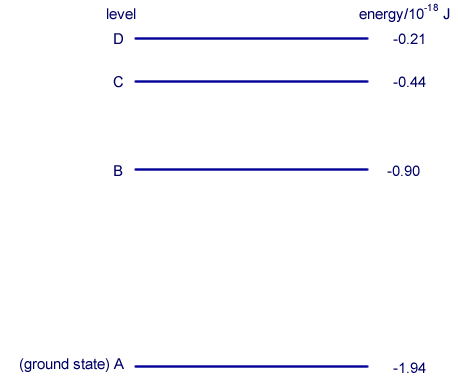
Wave/particle duality Q4. The diagram shows some of the electron energy levels of an atom.
An incident electron of kinetic energy 4.1 × 10–18 J and speed 3.0 × 106 m s–1 collides with the atom represented in the diagram and excites an electron in the atom from level B to level D.
(4 marks)
(3 marks) (Total 7 marks) |
Follow me...
|