Quantum Phenomena - discrete energy levels for electrons
Q6. The diagram below shows some of the energy levels for an iron atom.
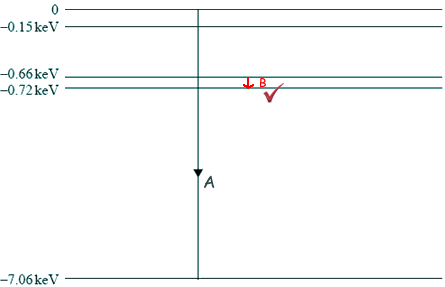
(i) Draw another arrow on the diagram above to represent the smallest energy change possible for an electron moving between two of the energy levels shown. The electron energy change selected must result in energy being emitted from the atom. Label this arrow B.
See diagram
(1 mark)
(ii) In the diagram, when the energy change labelled A occurs an X-ray photon is emitted. Show that the frequency of the photon is approximately 2 × 1018 Hz.
ΔE = 7.06 keV
ΔE = 103  x 1.6 × 10–19
x 1.6 × 10–19  x 7.06 J
x 7.06 J
E = hf
f = E/h = 1.6 × 10–16 x 7.06/ (6.63 × 10–34) Hz
f = 1.7 x 10-18 Hz
which rounds to 2 × 1018 Hz.
(3 marks)
(Total 4 marks)


