A level: Kinetic Theory Questions
Q2.
(a) Use the kinetic theory of gases to explain why:
(i) the pressure exerted by an ideal gas increases when it is heated at constant volume,
When the gas is heated the average speed of molecules increases. therefore there are more collisions (with the wall) per second and more momentum change per collision.
and more momentum change per collision.
This results in a
greater force per unit area on the container walls (greater pressure) because there is an increase in momentum change per second  producing a greater force (Ft =
producing a greater force (Ft =  p).
p).
(ii) the volume occupied by an ideal gas increases when it is heated at constant pressure.
When the gas is heated the average speed of molecules increases.
The faster molecules would result in more momentum change per collision, increasing the pressure, if the volume was kept constant.
However if the volume was greater, there would be fewer collisions per second. Therefore to maintain a constant pressure the volume of the gas must increase when the gas is heated.
(4 marks maximum)
(b) A quantity of 0.25 mol of air enters a diesel engine at a pressure of 1.05 × 105 Pa and a temperature of 27°C. Assume the gas to be ideal.
(i) Calculate the volume occupied by the gas.
27oC = (273 + 27) K = 300K
pV = nRT
V = nRT/p
= 0.250 x 8.31 x 300/(1.05 × 105) 
= 5.94 × 10–3 m3 
(ii) When the gas is compressed to one twentieth of its original volume the pressure rises to 7.0 × 106 Pa.
Calculate the temperature of the gas immediately after the compression.

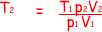
but V2/V1 = 1/20
so, T2 = 300 x 7.0 x 106/(1.05 x 105 x 20) 
= 1000 K 
T = pV/nR
T = 1000 K 
(4 marks)
(Total 8 marks)






